|
#1
26th September 2012, 07:06 PM
|
|||
|
|||
What is the difference between Ideal Gas and Real Gas?
diffrence b/w ideal gas and real gas?
|
|
#2
26th September 2012, 07:49 PM
|
|||
|
|||
|
the ideal gas will have a equilibrium conditions such as it's pressure, volume or temperature. but a real gas doesn't need to be equilibrium and satisfy the conditions. there can be change in pressure, the content of elements, volume, etc.
hence there is a difference in ideal gas & real gas. |
|
#3
26th September 2012, 07:54 PM
|
|||
|
|||
|
Hello Friend,
Ideal gas has no volume, and no forces of attraction between the molecules. it obeys PV =nRT But a real gas has volume, and no forces of attraction between the molecules, and it does not obey PV=nRT Hope this helps you . Thank You.. |
|
#4
26th September 2012, 08:35 PM
|
|||
|
|||
|
Ideal gas-An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.
At normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Z=PV/RT=1 i.e z(compressibility)=1 Many gases such as air, nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances Real gas-Many gases such as air, nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Real gas behavior is actually complex. real-gas models have to be used near the condensation point of gases, near critical points, at very high pressures, and in other less usual cases. The value of compressibility is not equal to 1. It may be higher or lower than this. Thanks |
|
#5
26th September 2012, 08:38 PM
|
|||
|
|||
|
hai,
You are asked the difference between Ideal gas and Real Gas. So some of information giving as per my knowledge. *The states of matter are liquid,solid,and gas which can be recognized through their key characteristics. *The Behavior of real gases is very much complex while the behavor of ideal gases is much simpler SUMMARY: 1.Ideal gas has no definite volume while real gas has definite volume. 2.Ideal gas has no mass whereas real gas has mass. 3.Collision of ideal gas particles is elastic while non-elastic for real gas. 4.No energy involved during collision of particles in ideal gas. Collision of particles in real gas has attracting energy. 5.Pressure is high in ideal gas compared to real gas. 6.Ideal gas follows the equation PV=nRT. 7.Real gas follows the equation (P + a/V2) (V b) = nRT. regards.........santhosh................ |
|
#6
26th September 2012, 10:32 PM
|
|||
|
|||
|
any name associated with the word 'ideal' means to have standard characteristies for that particular thing. For example-'ideal gas' means a gas which has the standard characteristics like oxygen in 'ideal air' is exact 21%. but when 'real' is associated with it, i.e. when we say 'real gas' it means the form in which it actually exists. hence the 'ideal gas' will be the purest for of gas having standard characteristics while the 'real gas' will be somewhat contaminated impuring its characteristics and having slight or more variations than ideal gas. in practice, 'ideal gas' is theoritical concept. no ideal gas actually exists. the one which exists is called as the 'real gas'.
|
|
#7
26th September 2012, 11:08 PM
|
|||
|
|||
|
HI dear..........
The difference between Ideas Gas & Real Gas: IDEAL GAS: An ideal gas is a theoretical gas composed of a set of randomly-moving point particles that interact only through elastic collisions. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics. The ideal gas model tends to fail at lower temperatures or higher pressures, when intermolecular forces and molecular size become important. At some point of low temperature and high pressure, real gases undergo a phase transition, such as to a liquid or a solid. The model of an ideal gas, however, does not describe or allow phase transitions. These must be modeled by more complex equations of state. REAL GAS: Real gas, as opposed to a Perfect or Ideal Gas, effects refers to an assumption base where the following are taken into account: Compressibility effects Variable heat capacity Van der Waals forces Non-equilibrium thermodynamic effects Issues with molecular dissociation and elementary reactions with variable composition. For most applications, such a detailed analysis is "over-kill" and the ideal gas approximation is used. Real-gas models have to be used near condensation point of gases, near critical point, at very high pressures, and in several other less usual cases  Good luck........... |
|
#8
27th September 2012, 04:47 AM
|
|||
|
|||
|
Dear Friend,
The Difference between ideal gas and real gas is that : Ideal Gas follows the equation [ PV = nRT] whereas Real Gases doesn`t follow this equation. They follow the equation [P+ an2/v2 = nRT] This is the information i have to the best of my knowledge regarding your query. Hope the information will help you to the extent you want. |
|
#9
27th September 2012, 03:21 PM
|
|||
|
|||
|
Difference Between Ideal Gas & Real Gas
 An ideal gas is a monotonic gas with no potential electronic interaction with its neighbors.An ideal gas molecule has mass and velocite (temperature) but no volume. It does not condense, nor does it have a triple point. Helium comes is the closest to being an ideal gas. 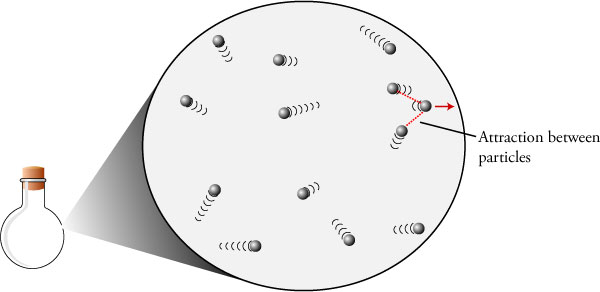 Real gasses condense, have electronic interactions, mass, velocity (temperature), and volume. It both condenses and sublimates and has a triple point. Many real gasses behave like ideal gasses over a wide pressure and temperature range. Ideal gasses obey PV=nRT where Pressure, Volume, Temperature, and molal inventory are variables and R is a gas constant. This allows comparisons to other ideal gasses by P1V1(n1T1)=P2V2(n2T2) Many other real gas laws exist to compensate for the non-ideal behavior of real gasses |
|
#10
29th September 2012, 12:37 AM
|
|||
|
|||
|
Ideal Gas ------------------ Real Gas >> Ideal gas has no definite volume ------------------ >> Real gas has definite volume >> Collision of ideal gas particles is elastic ------------------ >> Non-elastic for real gas >> Ideal gas has no mass ------------------ >> Real gas has mass >> Ideal gas follows the equation PV=nRT ------------------ >> Real gas follows the equation (P + a/V2) (V b) = nRT All the Best With Regards... Dharm |
|
#11
30th September 2012, 12:24 AM
|
|||
|
|||
|
HERE IS DIFFERANCE:
>> Ideal gas has no definite volume >> Real gas has definite volume >> Collision of ideal gas particles is elastic >> Non-elastic for real gas >> Ideal gas has no mass >> Real gas has mass >> Ideal gas follows the equation PV=nRT >> Real gas follows the equation (P a/V2) (V ¨C b) = nRT |
|
Related Topics: |
||
| Thread | Replies | Last Post |
| Can I go for BDS course on the basis of 12th class marks or on the basis of B.Sc being a candidate of about to complete B.Sc in Medical Technology? | 2 | 12th February 2015 12:10 AM |
|
|